PR
News
notice
Growing importance of artificial platelets, a collaboration of industry, schools, research institutes and hospitals to develop and commercialize them
2023.04.05
Growing importance of artificial platelets, a collaboration of industry, schools, research institutes and hospitals to develop and commercialize them

Industry, schools, research institutes, and hospitals have joined forces to develop and commercialize 'artificial platelets' to solve the platelet shortage in Korea.
Due to the aging society and the epidemic of infectious diseases such as COVID-19, the number of blood donors is decreasing, causing a shortage of platelets in the medical field. Platelets, which can only be supplied through blood donation, are the main component of hemostasis in the blood and are made from megakaryocytes in the bone marrow, and are applied to emergency transfusions and treatment of thrombocytopenia caused by chemotherapy or drug treatment.
Platelets can also be used in various regenerative medicine applications. Recently, the government is also promoting R&D projects using stem cells for artificial blood and artificial platelets.
To solve the platelet shortage, it is necessary to develop artificial platelets and provide a sufficient supply. (hereinafter referred to as Ducell Bio), a leading domestic company in the development of artificial platelets, was formed and launched on the 5th of this month to develop and commercialize artificial platelets.
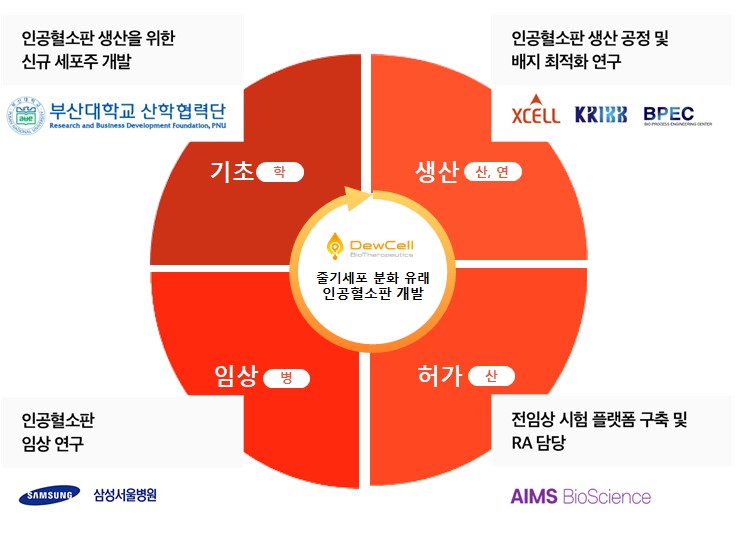
The consortium includes Ames Bioscience, Xcell Therapeutics, Pusan National University, Korea Biotechnology Institute's Center for Biocommercialization, and Samsung Medical Center's Institute for Cell and Gene Therapy.
Dussell BioTherapeutics is developing a technology that induces the differentiation of induced pluripotent stem cells (iPS) into hematopoietic stem cells to make megakaryocytes, which are then used to make platelets.
"The project to develop and commercialize human stem cell-derived artificial platelets is an item that can satisfy unmet needs in the medical community at a time when blood supply is not smooth," said Lee Min-woo, CEO of DusselBio. "Through the formation of this consortium, we hope to establish an industry-academia-university-industry partnership system and rapidly proceed with the development and mass production of artificial platelets and their commercialization through synergies resulting from the collaboration of specialized institutions in each field.
Dusselbio will play a central role in developing and producing human stem cell differentiation-derived artificial platelets, establishing analytical methods and confirming in vitro and in vivo efficacy, and has signed a business agreement (MOU) with each institution to develop artificial platelets in advance to form the consortium and coordinate the R&R of each institution.
In this consortium, Pusan National University's Industry-University Cooperation Center (Prof. Heo Jin, College of Medicine) was responsible for developing a new cell line that can stably produce artificial platelets by applying stem cell differentiation technology and gene manipulation technology. He is currently working on inserting various genes that can increase platelet production yield into the induced pluripotent stem cell (iPSC)-derived artificial platelet production cell line developed by Ducell Bio, and developing new cell lines that can produce artificial platelets from tissue-derived stem cells other than iPSCs.
The BioCommercialization Support Center of the Korea Biotechnology Research Institute (Center Director Eun-Kyo Lee) is in charge of developing production processes to realize economical artificial platelet production. It will support research on optimizing production processes such as cell culture and purification for mass production of artificial platelets based on public infrastructure that can support the advanced bio industry.
Xcell Therapeutics will be responsible for the development of culture media, which is essential for research on optimizing the production process of artificial platelets. Ltd. is the only company in Korea that develops and produces culture media for advanced bio-industries, and plans to develop platelet culture media based on its technology that developed the world's first serum-free chemically defined media in this consortium.
Serum-free chemically defined media are recognized by the industry as the next generation of culture media in terms of stability, cost-effectiveness, and reliability. Given the nature of platelets, which are to be directly injected into the human body, large-scale culture and stability are essential, and the use of chemically defined serum-free media is the only alternative, according to the company.
"Based on our world-class technology for developing 'serum-free chemical composition media' for the advanced biotechnology industry, we will succeed in developing optimized media for artificial platelet cell lines," said Dr. Lee Yi-il, CEO of Xcell Therapeutics.
Ames Bioscience, a non-clinical and clinical consulting company, will support the IND submission and approval of artificial platelets for clinical entry. "In order to conduct the first clinical trial of stem cell differentiation-derived artificial platelets in Korea, it will be necessary to discuss and coordinate many aspects with the Ministry of Food and Drug Safety, but it is a necessary task that someone must do, so we joined this consortium with a spirit of challenge and pioneering," said Dong-Seok Lim, CEO of Ames Bioscience.
Samsung Medical Center will be responsible for establishing a TPP (Target Product Profile) for the development of artificial platelets and conducting clinical trials for commercialization. Dr. Gunhee Yoo, a pediatric hematologist-oncologist at Samsung Medical Center, believes that the stem cell-derived artificial platelets developed by Ducell Bio could be an alternative solution to complement the supply of blood-derived platelets that many patients need in the real world. Dr. Yoon-Sil Jang, director of the Institute for Cell and Gene Therapy, believes that by identifying clinical needs from the early stages of development and establishing standards to evaluate the efficacy and function of platelets, it is possible to achieve rapid commercialization.
"Although the consortium started with the participation of six institutions, the development of artificial platelets is a concentration of advanced biotechnology and requires the collaboration of many specialized institutions," said an official from Ducell Bio. "To this end, we will continue to create opportunities for collaboration in various fields with institutions that are willing to join the consortium."
Meanwhile, the global platelet market is expected to reach KRW 6 trillion by 2026, according to Global Market Insights. The demand for platelets is expected to grow as the rate of blood transfusions for chronic blood diseases and surgery increases.
Source : Biotimes (http://www.biotimes.co.kr)
