PR
News
notice
If large, medium, and small companies and governments work together, they can "make biomaterials, components, and equipment self-reliant
2021.04.12
If large, medium, and small companies and governments work together, they can "make biomaterials, components, and equipment self-reliant
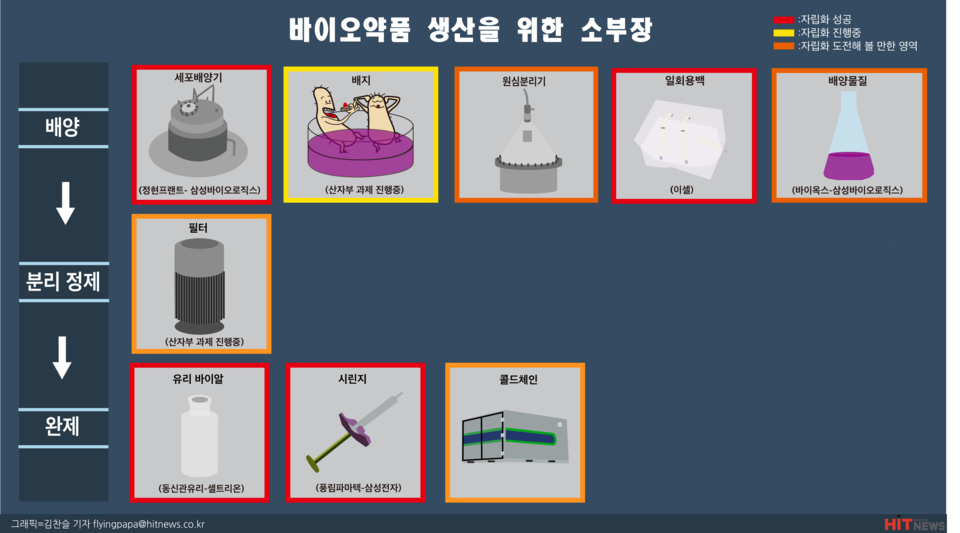
The production process of biopharmaceuticals consists of cell culture, separation, purification, and finalization. Each of these processes requires different materials, parts, and equipment. If we look at the sub-divisions of the cell culture stage, the first step in the production of biopharmaceuticals, we can see that in order to grow cells, or in other words, to grow cells, we need a cell culture machine, which is an equipment that helps cells multiply in optimal conditions, and a medium that corresponds to their food.
Major companies that supply cell culture machines include Thermo Fisher, Merck, and GE Healthcare in the United States. Last year, Samsung Biologics signed a memorandum of understanding (MOU) with Jung Hyun Plant, a company specializing in the manufacturing of cell culture machines in Korea. According to the MOU, Samsung BioLogics will supply all the various types of incubators for the 4th plant from Jung Hyun Plant. Previously, in 2016, Samsung BioLogics signed a contract with domestic company Vioxx to supply cleaners and disinfectants for cleaning inside production facilities, starting the process of self-reliance in the bio sub-department.
While cell cultivators are becoming more self-reliant, the company still relies entirely on imports of medium, which is the food for cells. Currently, Thermo, Cytiva, Merck, and Lonza are known to hold a large share of the media market.
In order to maintain their strong position in the market, these companies have not registered any patents on the production of the media, in order to avoid disclosing their production methods, including the composition of the media, to the outside world.
As one biopharmaceutical industry insider put it, "It's one of the most difficult areas to become self-reliant, because there's very little disclosure about how we do it." Fortunately, the Ministry of Trade, Industry and Energy is reportedly working on a national initiative to make media self-sufficient.
Other parts and equipment needed in the cell culture stage include centrifuges and disposable bags. All of these are currently produced by overseas companies.
Filter supply anxiety sparked by Japan whitelist crisis
Celltrion and Dongshin Tube Glass create success stories in vials
After cells have been grown and cultured to the desired number, filters are needed to separate and purify foreign substances such as viruses. Filters are divided into microfiltration (MF) filters and nanofiltration (NF) filters. In particular, among the 1194 items on Japan's 'white list' against South Korea in 2019, the biopharmaceutical industry included Ashikasei's virus removal filter Planova, which disrupted domestic biopharmaceutical production.
"Along with media, filters are an area where it is difficult to become self-reliant," said an industry insider, "There are a few companies that can produce paper (for filters), but it is expected to be difficult to meet regulatory production conditions."
After the separation and purification process, the finished product needs to be packaged in glass vials or, in the case of injectable items, syringes, etc. In this process, there are cases of collaboration between large and small companies to achieve small-scale self-reliance. These are the success stories between Celltrion and Dongshin Tube Glass Industry and Samsung Electronics and Punglim Pharmatech.
Celltrion dispatched a Celltrion employee to Dongshin Kwan Glass for about two years to help with documentation to meet the drug regulatory environment.
"By using products produced by Dongshin Kwan Glass instead of overseas products, we were able to lower our production costs by about 20 billion won," said Celltrion Chairman Seo Jeong-jin at the launching ceremony of the Bio Sector Solidarity and Cooperation Council at the Marriott Hotel in Yeouido last September.
Usually, glass vials for pharmaceuticals are sold by the German company Schott, which is manufactured in Indonesia. While it is possible to produce glass vials in Korea that are as good as those produced in Indonesia, switching vials is not as easy as it sounds.
"In order to change the vials (to products developed by a domestic company), the company name, size, etc. must be changed, and it takes about 24 months to produce other safety test data," said an industry insider, explaining that such a procedure costs about 2 to 5 billion won.
Punglim Pharmatech, in collaboration with Samsung Electronics, has developed a syringe manufacturing technology that applies minimum dose volume (LDS) technology to minimize the amount of injection remaining per dose to 4 μL, compared to 84 μL or more for ordinary syringes. Therefore, while ordinary syringes can only provide five doses per bottle of COVID-19 vaccine, Punglim's LDS syringes can provide more than six doses per bottle.
Funglim's LDS syringes have passed performance tests such as the minimum injection volume of US pharmaceutical companies and met the performance requirements of COVID-19 vaccine manufacturers. The company has also applied for domestic technology patents and design patents related to the minimum injection volume and safety protection guards, and is in the process of applying for international patents such as the US and EU.
Punglim Pharmatech's rapid commercialization has been supported by Samsung and Samsung Biologics. On December 24 last year, Samsung Electronics provided Punglim Pharmatech with 30 manufacturing experts, including its own facilities, to support mass production, including the introduction of smart factories.
In this process, it is said that the production of prototype molds and prototypes were completed in just four days during the year-end and New Year holidays through Samsung's Gumi and Gwangju partner factories. We also helped the company overcome various regulations from the Ministry of Food and Drug Safety.
In addition, the cold chain, which requires distribution of vaccines and other products at relatively low temperatures, is also classified as a small business. The importance of the cold chain is very high, as it is estimated that around 10% of the cost of biopharmaceuticals is spent on cold chain transportation. In fact, Turkish airline Cargo has entered the cold chain industry by transporting COVID-19 vaccines manufactured by Sinovac.
Turkish Airlines Cargo, which has been transporting medical supplies and equipment related to COVID-19, transported the first batch of COVID-19 vaccines procured by the Turkish Ministry of Health from Beijing to Ankara, Turkey, in 17 dedicated cold containers on a dedicated flight. In addition to the vaccines, Turkish Airlines Cargo has been expanding its pharmaceutical supply chain, increasing its cargo capacity by 30% since COVID-19, with original cold chain shipments reaching 25,000 tons (t) per month.
Through its 'TK Pharm' service, Cargo has been actively engaged in the cold chain of vaccines and other pharmaceuticals. Currently, it has formed a global pharmaceutical and distribution network with major regional bases such as Mumbai, Brussels, Istanbul, Singapore, Dubai, Basel, London, and Amsterdam, enabling pharmaceutical cargo services to more than 300 locations.
"The cold chain is very important, accounting for 10% of the unit cost of biopharmaceuticals," said an industry insider, adding, "Based on Turkey's example, Korean airlines should also look into this business."
To summarize, the areas where small-scale self-reliance for biopharmaceutical production is possible are glass vials, syringes, cell cultures, and disposable bags. Furthermore, filters, cold chains, and media can be prepared for long-term self-reliance.
An industry insider said, "Self-reliance in the first stage of the biopharmaceutical production process, the culture stage, is relatively difficult, while self-reliance in the finished product stage can be challenged by domestic companies first."
Government regulatory support and consultation are key to self-reliance
A win-win alliance between large and small companies is essential
While there are some examples of small-scale self-reliance, there is still a long way to go, especially when it comes to adapting small-scale technologies to pharmaceutical production regulations, which requires government support and cooperation between large and small companies.
In fact, there is a case where an attempt to make glass slides for biopharmaceutical production failed not because of the technology, but because of the lack of documentation to comply with drug regulations.
Dongshin Kwan Glass, Jeonghyeon Plant, and Punglim Pharmatech, which are considered successful cases of self-reliance, would not have been possible without the active cooperation of large companies such as Celltrion and Samsung Biologics.
In the case of Dongshin Kwan Glass, Celltrion's dispatched experts, who are familiar with the regulatory science of pharmaceuticals, stayed for two years and helped with all the relevant paperwork. The Jeonghyeon plant was also able to develop at a faster pace because it built the facility from the beginning of the expansion of Samsung Biologics' Plant 4.
"SMEs that succeeded in becoming self-reliant were able to commercialize at a faster pace because they received support from large companies (Celltrion, Samsung Biologics, etc.) from the beginning of their technology development and documentation to meet drug regulatory standards," said an industry insider. "Most small and medium-sized technology development SMEs often face difficulties because even though they have the technology, they have not established a regulatory science concept that meets the standards of the Ministry of Food and Drug Safety and the FDA."
In fact, in the case of Punglim Pharmatech, the company was able to obtain approval from the Ministry of Food and Drug Safety within a month of development, and the FDA approved it in about eight weeks. This is why the cooperation of the Ministry of Food and Drug Safety is important for the self-reliance of small businesses in the future.
Last year, the Ministry of Trade, Industry and Energy announced that it will provide 85.7 billion won over the next five years for the development of 16 small parts such as filters and media.
In addition, the 'Bio Small Parts Cooperation Council' was established to strengthen the competitiveness of bio small parts. The council is composed of 13 demanding companies, such as Celltrion and Samsung Biologics, and 42 suppliers, such as Amicogen, Dongshinkwan Glass Industry, EcoNiti, and J-OTECH. The Korea Biotechnology Association, the Korea Biopharmaceutical Association, and the Korea Institute of Industrial Technology Assessment and Management support the council's operations.
The organizations participating in the council plan to start by cooperating on technology development in bio-core sub-divisions, and once the supplier companies have developed items at a level that meets the needs of the demanding companies, the demanding companies will support them with demonstration tests and technical advice. In addition, the Korea Bio Association, which runs the council, will listen to the companies' pain points and provide various consulting and information needed by the demanding companies.
"It is important for large companies such as Celltrion and Samsung Biologics to use the small parts developed by domestic companies, but it will also be important for universities and government-funded research institutes to use the products of domestic companies to build references in their papers," explained an industry insider.
Source : Hit News (http://www.hitnews.co.kr)
