PR
News
notice
Xcell Therapeutics Expands Production Capacity to 2 Million Biotransport Media Per Month
2020.11.25
Xcell Therapeutics Expands Production Capacity to 2 Million Biotransport Media Per Month
VTM automation system for large-scale production of transportation badges
ISO 9001-CE certification for cGMP plant... Started delivering to exporters

Korean biotechnology company Xcell Therapeutics has established a large-scale mass production system by introducing a transport medium (VTM) automation device. The company has established a domestic production infrastructure for bio products essential for the prevention of the novel coronavirus infection (COVID-19).
According to the bio industry on the 25th, Xcell Therapeutics will be able to produce VTMs at a scale of up to 2 million pieces per month with the introduction of this automated equipment.
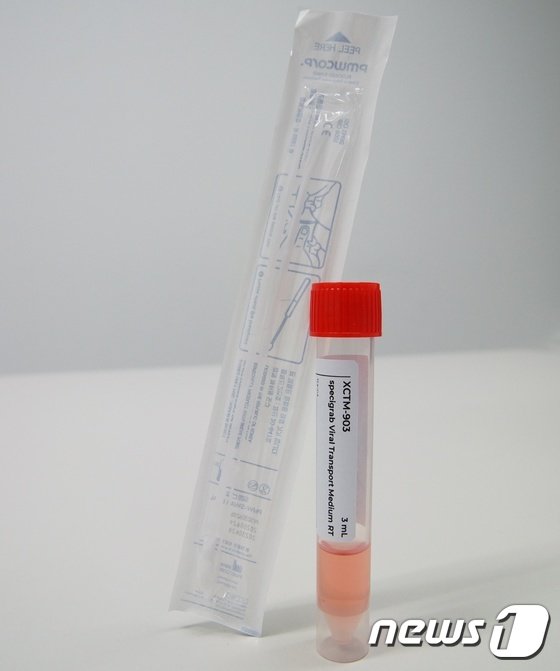
Viral Transport Media (VTM) refers to a medium for transporting viral clinical specimens. COVID-19 is tested by inserting two medical swabs, each about 20 centimeters long, deep into the back of the nose and throat to collect secretions, then combining the swabs into a single tube and transporting it to a laboratory for testing.
The tube contains a liquid reagent that allows the COVID-19 virus to survive for at least 48 hours, which is the transport medium.
Xcell Therapeutics is the first company in the world to develop a chemically composed serum-free medium for stem cells that is cGMP-grade. After being recognized for its technology both domestically and overseas, the company has built and operated a cGMP-grade production plant in Yongin.
Xcell Therapeutics has been preparing for the development and production of transport media since May, when the COVID-19 epidemic was largely centered in the capital region, and has recently been delivering to overseas exporters in earnest.
Transport medium is a biological product whose demand is exploding in the twin epidemic situation of COVID-19 and influenza (flu). In particular, product quality is critical every week. If the quality of the transport media used to move specimens, including the COVID-19 virus, to analytical instruments is compromised, it could wreak havoc on the epidemic response.
Currently, Xcell Therapeutics is ISO 9001, ISO 13485, and CE certified for its transport media. ISO 9001 is an international standard for quality management systems established by the International Organization for Standardization (ISO). Products and service requirements are objectively evaluated by a third-party certification body to determine certification status.
"We will provide products that guarantee high reliability through the world's best culture medium manufacturing technology and cGMP production facilities," said Dr. Lee Yi-il, CEO of Xcell Therapeutics. "We are currently working on a project to develop new products that are safer and more reliable than the current ones with an authority in the bio field in the United States."
"Currently, the demand for transportation badges is continuously increasing due to the COVID-19 pandemic," said Mr. Lee. "Now that we have the infrastructure to compete with companies around the world, we will speed up our expansion into the global market as well as K-prevention."
"If the demand for our products increases, we have a system in place to respond by expanding our fish facilities in Yongin or Osong, Chungcheongbuk-do," said Lee.