Technology
CellCor™
-
01
Next
Generation Media- Development of true animal-origin free chemically defined media by replacement of recombinant proteins with small molecules
-
Development of chemically defined media for different cell types
1) Dermal Papilla cells
2) Keratinocytes
3) NK/T cells
-
02
Clonal
Selected MSCs- Acquire high purity MSCs by clonal selection method
- Create next-generation platform of MSC clones with various characteristics
-
03
MSC-derived
Exosomes- Develop mass exosome cultivation platform utilizing our chemically defined media
- Develop mass uniform exosome cultures through profiling and characterization
-
04
Automated Mass
Cell Culture Platform- Secure safety, stability and economic feasibility data
- Setting standards for next-generation cell therapy
- Automated mass culturing of cells and exosomes
- Develop core technology for 3D bio-printing and functional cosmetics
Next Generation Media
defined Media
Fully Chemically
Defined Media for MSCs
- Replace recombinant proteins with small molecules
- Truly animal origin-free
- All compositions are chemically defined
- Advancing media technology to the next level
- Expected to be completed by year 2022
Dermal Papilla
Cell Culture Media
- Developing media based on patented follicle growth materials
- Serum-free defined media
- Expected to be completed in the year 2020
Keratinocyte
Culture Media
- Developing media specific for keratinocytes
- Serum-free media
- Co-developing with Company X
- Expected to be completed in the first half of year 2020
NK cell/T Cell
Culture Media
- Developing media by applying specific immune cell factors.
- Initiated in October 2019
- Expected to be completed in late 2021
Clonal Selected MSCs
-
Mesenchymal Stem Cell
-
Clonal Selection
Single Cell

-
Mesenchymal Stem Cell

Acquisition and research of homogeneous
monoclonal stem cells using CellCor™.
Acquisition of High Purity Stem Cells
-
01
Stem cell library
Establishment of a high-purity clonal stem cells library
-
02
Stem Cell Therapy
Provide specific targeted high-purity stem cells for the development of stem cell therapy
Automated Mass Cell Culture Platform
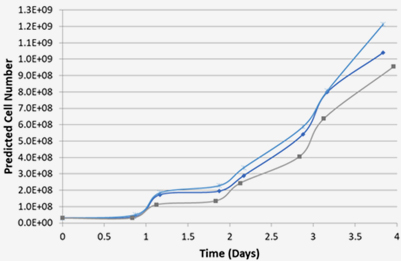
MSC T175 Culture (+p2)
Bioreactor Expansion
(+P3, 4 days)Cell Characterization
-
MSC

-
Static Culture

-
4days(~20 folds)

-

3D printing
-

Research
-

Material
-

Exosome
MSC-derived Exosomes
MSC cultured
with CellCor™Mass Production
Cultured
Media SeparationExosome
isolation using
B company’s
productCharacterization
and profiling

