Product
CellCor™
Cell culture is crucial in the research, development, and production of therapeutics in the health care industry. Efficacy, sensitivity, and reproducibility are the key attributes needed for optimal media. After many years of research, Xcell Therapeutics has developed the CellCor™ line of MSC cell culture media products, which began with development of a serum-free defined formulation, and evolved into the launch of our serum-free TRUE chemically defined media. Our CellCor™ media provide superior performance while maintaining strong safety and quality assurance through our strict adherence to good manufacturing practices.
CellCor
200+ Compounds
Optimal Composition
Mass Production Process
Product |
CellCor™ SFD MSC |
CellCor™ CD MSC |
|---|---|---|
Class |
Serum-Free Fully Defined |
Serum-Free Chemically Defined |
Cell Type |
Human Mesenchymal Stem Cell | |
Advantages |
Proliferation↑ Homogeneity↑ |
Stemness↑ Genetic Stability↑ |
Product |
CellCor™ SFD MSC |
|---|---|
Class |
Serum-Free Fully Defined |
Cell Type |
Human Mesenchymal Stem cell |
Advantages |
Proliferation↑Homogeneity↑ |
Product |
CellCor™ CD MSC |
|---|---|
Class |
Serum-Free Chemically defined |
Cell Type |
Human Mesenchymal Stem cell |
Advantages |
Stemness ↑Genetic Stability ↑ |
Xell Therapeutics Catalog number
| Catalog No. | Product | Grade | Volume |
|---|---|---|---|
| YSP001 | CellCor™ CD MSC | RESEARCH (USE ONLY) GRADE | 500mL |
| YSP002 | CellCor™ CD MSC | GMP GRADE | 500mL |
| YSP009 | CellCor™ SFD MSC | RESEARCH (USE ONLY) GRADE | 500mL |
| YSP010 | CellCor™ SFD MSC | GMP GRADE | 500mL |
| Catalong No. | YSP001 |
|---|---|
| Product | CellCor™ CD MSC |
| Grade | RESEARCH (USE ONLY) GRADE |
| Volume | 500mL |
| Catalong No. | YSP002 |
|---|---|
| Product | CellCor™ CD MSC |
| Grade | GMP GRADE |
| Volume | 500mL |
| Catalong No. | YSP009 |
|---|---|
| Product | CellCor™ SFD MSC |
| Grade | RESEARCH (USE ONLY) GRADE |
| Volume | 500mL |
| Catalong No. | YSP010 |
|---|---|
| Product | CellCor™ SFD MSC |
| Grade | GMP GRADE |
| Volume | 500mL |
Xcell Classification of Media
Xcell Classification of Media
| Media | Abbreviation | May Contain | Does not Contain |
|---|---|---|---|
| Serum-Based |
- |
- |
|
| Serum Free |
SF |
does not contain
|
can contain
|
| Xeno Free |
XF |
does not contain
|
|
|
Animal Component Free Chemically-Defined |
ACFCD |
|
|
-
- -
- animal/human sera (i.e. FBS)
SF
- no serum/plasma from animal/human
- animal/human derived extracts and/or lysates
XF
- SF + no animal derived extracts and/or lysates
- human derived extracts and/or lysates
ACF
- SF/XF + no extracts and/or lysates
- animal/human derived recombinants
CD
- SF/XF + no extracts and/or lysates
- animal/human derived recombinants
Under what conditions should the media be thawed?
What types of cells are applicable to CellCor™?
Are there any precautions when culturing MSCs?
Is coating necessary when culturing MSCs?
Superior
-
Superior Proliferation Rate
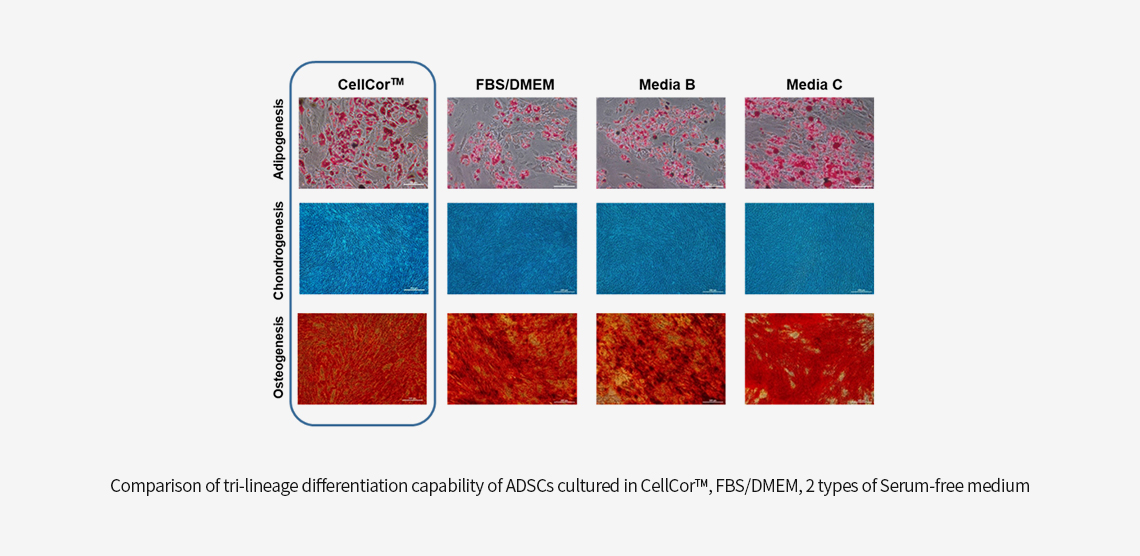
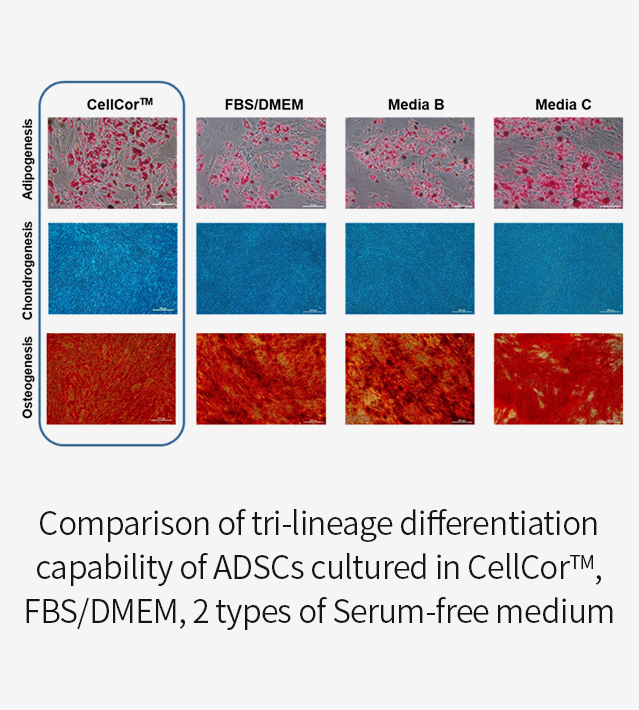
CellCor™ vs. FBS/DMEM vs. other Serum Free Media
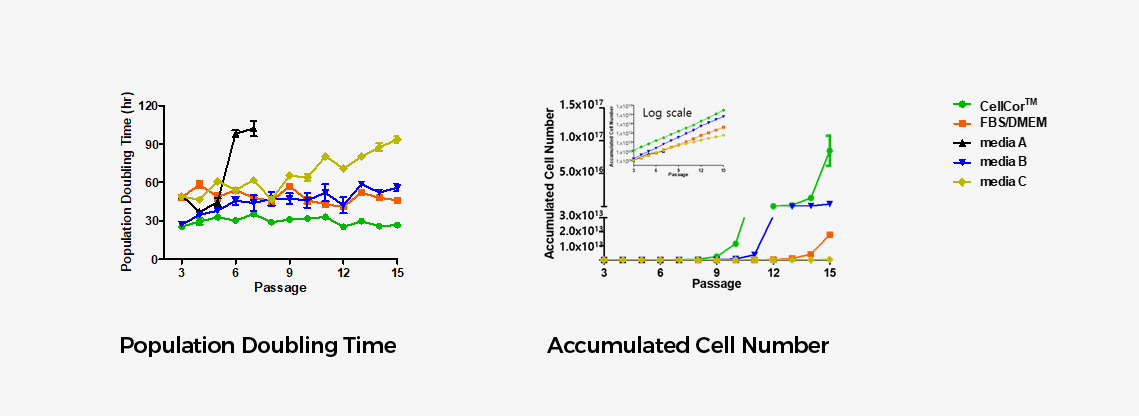
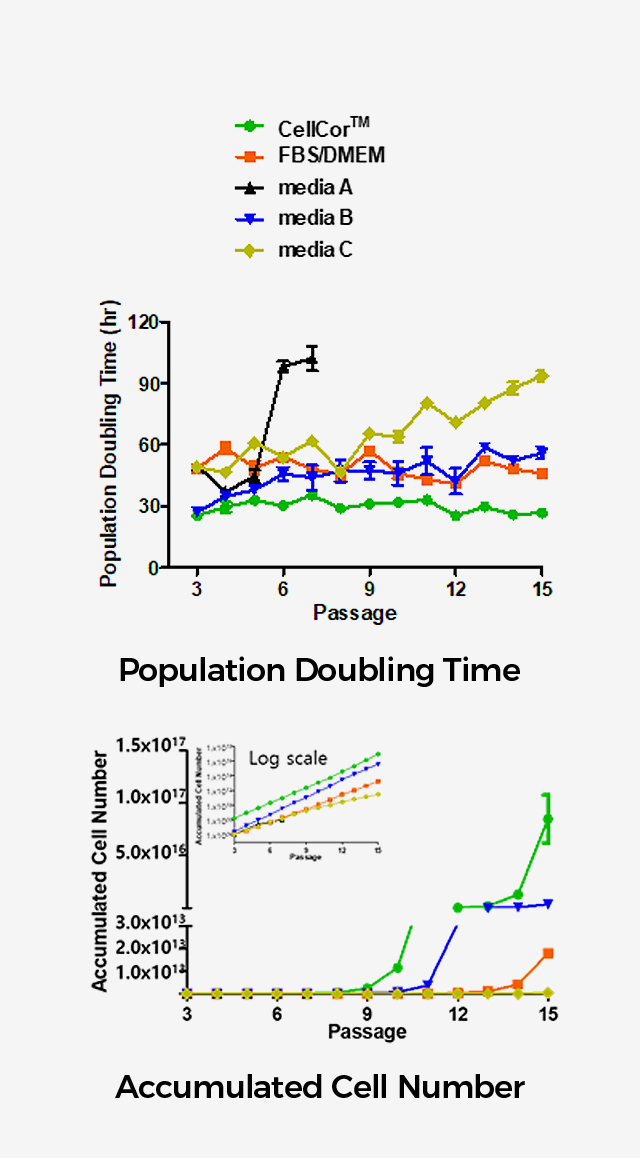
The major strength of CellCor™ is its superior proliferation rate.
CellCor™ maintained 20~31 hours during the PDT test for 15 passages while the competitions’ media maintained 60~80 hours and 100 hours at the longest.
Xcell has tested 3 competitors’ serum-free products along with FBS containing media available on the market . None of the 3 commercially available media supported better proliferation rate than CellCor™.
-
Surface Markers
MSCs can be characterized by surface markers set by International Society for Cellular Therapy guidelines. There are positive CD73, CD90, and CD105 and does not express CD14, CD34, CD45, and HLA-DR.
Surface Marker Comparison vs Other Serum Free Media
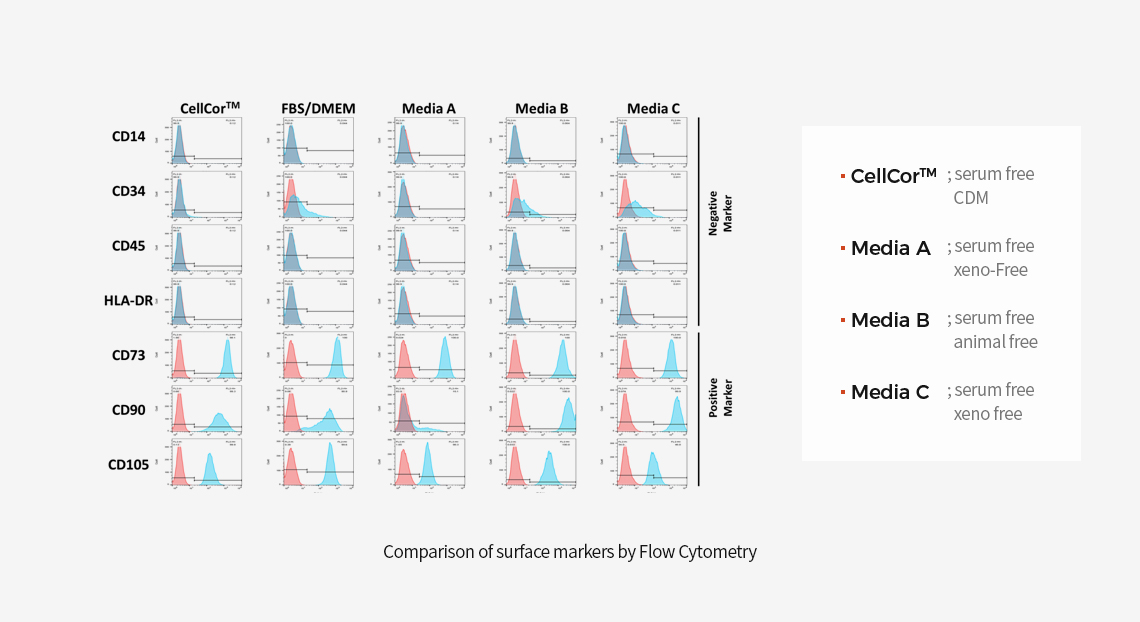
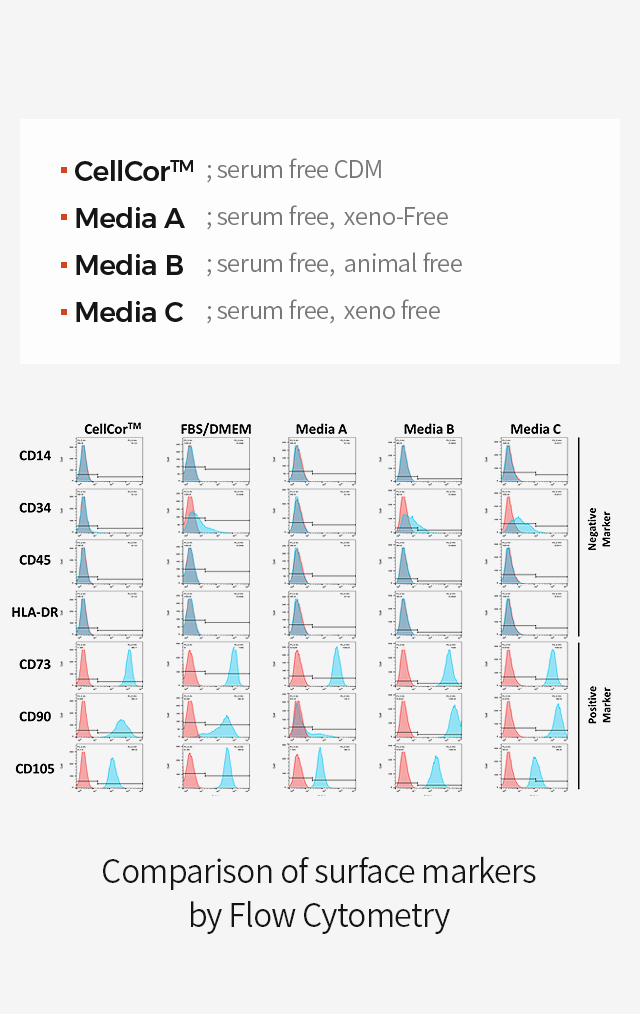
-
Superior Differentiation
The Mesenchymal stem cell is commonly demonstrated as Multipotent. The capability of differentiation is also the characteristic of MSCs. Tri-lineage differentiations are Adipocyte, Chondrocyte, and Osteocyte.